
Usually, mass is expressed in grams, but any unit of measure is suitable as long as you utilize an equivalent unit for both the component or solute mass and therefore the total or solution mass. Mass number 1 Atomic number 1 Relative atomic mass of hydrogen 1 Mass number 16 Atomic number 8 Relative atomic mass of oxygen 16 As there are 2 hydrogen atoms and 1 oxygen atom in a water molecule, the calculation is (2x1) + 16 18. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). Mass percent is used for representing the concentration of an element in a compound or a component in a mixture. The molar mass is used to convert grams of a substance to moles and is used often in chemistry. The mass number for all the elements is always a whole number. The mass of a mole of substance is called the molar mass of that substance.The mass number is written as a superscript on the left side of the symbol of the element.TIPS: Consult the Help page for details on using the peak search fields. CAVEATS: No UV data Raman peak searching not available.
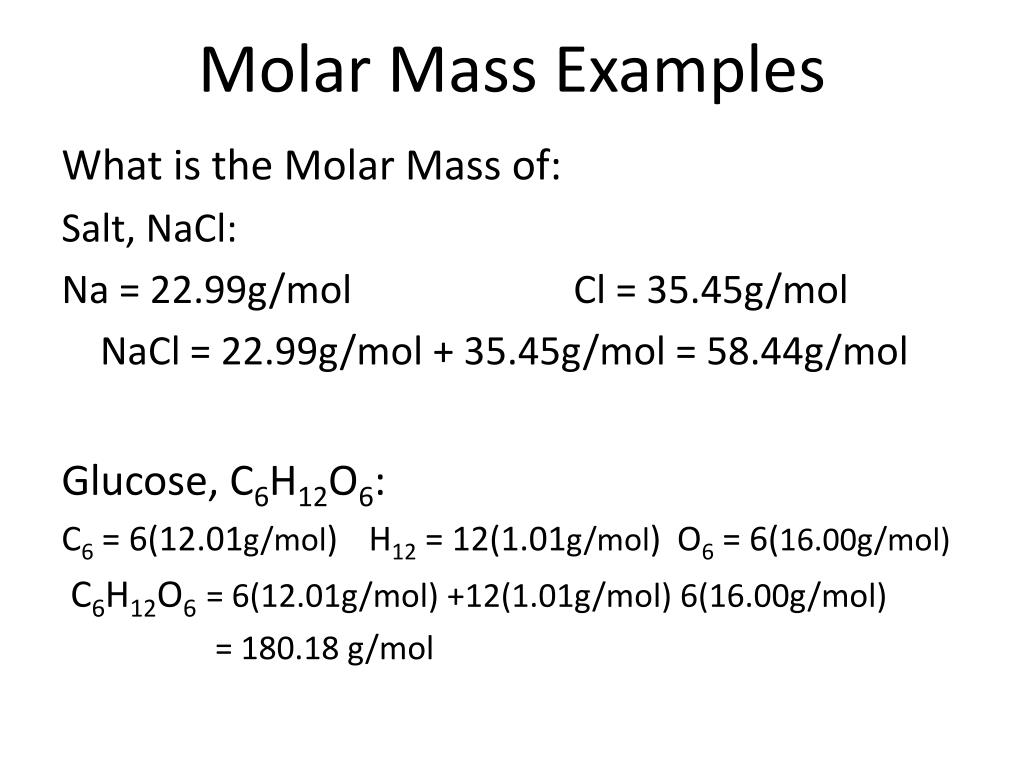
#Mass finder chemistry free#
The mass number allows us to tell the difference between elements with a different number of neutrons, called isotopes. DESCRIPTION: This free database from Japan allows searching by IR peaks, MS peaks and intensities, and 1H and 13C NMR shifts in ppm, along with element counts and basic identifiers. These are the primary carriers of electricity. Accepted entry includes: chemical name, InChI, InChI key, nominal mass, or molecular formula (Hill order) KnowItAll IR, Raman, and UV-Vis spectral libraries are not included in Compound Search at this time. While electrons are the subatomic particles like protons and neutrons but located outside the nucleus and are negatively charged. These are the constituent of the nucleus of all atoms except for hydrogen.

These have no net charge and have mass slightly more than that of protons. Just tap on the concept you need to be aware and you are ready to go. Neutrons are the subatomic particles present in atomic nuclei like protons. Online Tool of Chemistry saves you time and eliminates all your hassle while solving complex equations, or determining formulas. This dimensional analysis video tuto, tap Molar Mass Calculator) By. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. 02 x 10 web chemistry 51 1 stoichiometry calculation practice worksheet 1 calculate. The Center is located on the NIST main campus in Gaithersburg, MD. The IUPAC International Chemical Identifier (InChI TM) is a non-proprietary identifier for chemical substances that can be used in printed and electronic data sources thus enabling easier linking of diverse data compilations.It was developed under IUPAC Project 2000-025-1-800 during the period 2000-2004.
#Mass finder chemistry how to#
The molar mass of the molecular formula matches the molar mass of the compound.In this article, we will learn how to calculate mass number(A) but first off, lets define it. These products are intended to assist compound identification by providing reference mass spectra for GC/MS (by electron ionization) and LC-MS/MS (by tandem mass spectrometry) as well as gas phase retention indices for GC.


 0 kommentar(er)
0 kommentar(er)
